Introduction
Artificial Wombs are emerging at the cutting edge of reproductive medicine and neonatal care, offering a bridge between conventional neonatal intensive care and natural gestation. Artificial Wombs—also described in research as ectogenesis, extra‑uterine fetal incubation, and artificial amnion and placenta technology—aim to sustain extremely premature infants in a controlled physiological environment that closely mimics the natural uterus.
The potential of Artificial Wombs to improve survival outcomes for preterm infants and to transform neonatal medicine makes them a topic of intense scientific investigation, clinical interest, and ethical debate. As research accelerates toward first‑in‑human trials, it is vital to understand what artificial womb technology entails, how it works, and why it matters now.
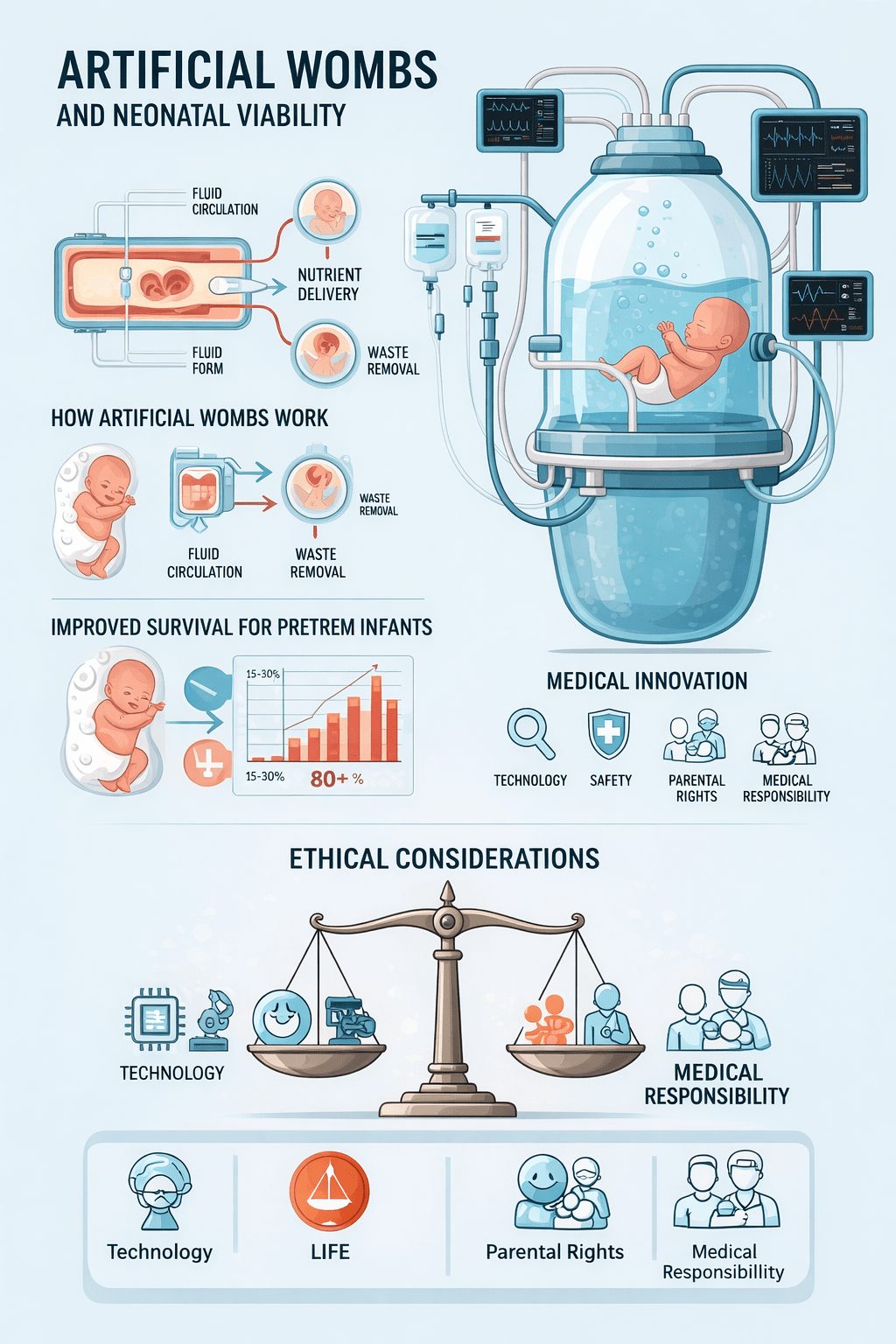
How Artificial Wombs Work
Fluid-Filled Chamber
The fetus develops inside a sterile, amniotic-fluid-like solution that mimics the natural uterine environment. This fluid supports organ development while protecting delicate tissues from stress and infection.
Artificial Placenta
A specialized device connects to the umbilical cord, delivering oxygen and nutrients while removing waste. This bypasses the infant’s underdeveloped lungs and provides stable metabolic support, mimicking maternal circulation.
Controlled Environment
Temperature, oxygen levels, and physiological parameters are continuously monitored and adjusted. This reduces risks associated with conventional incubators and ensures optimal conditions for extremely preterm infants.
Key Developments
-
EXTEND (Children’s Hospital of Philadelphia): Successfully supported premature lamb fetuses for weeks, allowing growth, weight gain, and eye opening—demonstrating feasibility for human translation.
-
Aqua Womb (Netherlands/Germany): A fluid-filled glass tank system currently preparing for clinical trials, aiming to support babies born at the limits of viability.
The Science of Artificial Wombs
Mechanisms and Physiology
Artificial womb technology replicates critical aspects of the maternal environment, including fluid‑based gestational conditions, nutrient delivery, and gas exchange. Devices such as the extra‑uterine environment for newborn development (EXTEND) and biobag systems use lab‑created amniotic fluid along with extracorporeal membrane oxygenation to sustain fetal physiology outside the maternal body. These systems simulate the protective, fluid‑filled environment of the uterus, maintaining sterile conditions while supporting organogenesis. This approach is often referred to as partial ectogenesis when used after early delivery and stands distinct from full ectogenesis, which would hypothetically encompass complete gestation outside a human uterus.
Impact on Neonatal Care
The primary clinical purpose of artificial wombs is to support infants born at the edge of viability (around 22–28 weeks gestation) who otherwise face high risks of respiratory distress, neurodevelopmental challenges, and mortality when cared for solely in traditional neonatal intensive care units. By preserving native fetal physiology in a fluid‑based environment rather than transitioning immediately to air‑filled lungs and external life support, artificial wombs can reduce the physiological shocks associated with premature birth. These systems have shown success in animal models, sustaining extremely preterm lambs’ development with outcomes comparable to age‑matched controls.
Clinical and Ethical Dimensions
Preclinical Validation and Translation
Research literature underscores both the promise and current limitations of artificial womb technology. Preclinical success has validated the physiological feasibility of sustaining development outside the womb, but significant engineering and clinical challenges remain, including umbilical cannulation, sterile fluid management, and long‑term developmental monitoring. Ethical frameworks and research roadmaps emphasize that moving toward human trials requires careful consideration of both medical risk and informed consent processes for guardians and clinicians.
Ethical and Legal Considerations
Artificial wombs raise complex ethical and legal questions that extend beyond technology to societal values and definitions of birth. Bioethical discourse addresses issues such as maternal autonomy, fetal personhood, and the ontological status of a fetus supported outside the body. Some ethical analyses challenge whether artificial gestation could transform legal frameworks surrounding reproductive rights, parental consent, and neonatal personhood. Ethicists also debate implications for gender dynamics in reproduction, potential inequalities in access to care, and the need to anchor innovation in human dignity.
Broader Implications and Future Directions
Research, Regulation, and Integration
Artificial womb technology sits at the crossroads of bioengineering, obstetrics, neonatology, and ethics. Ongoing research calls for robust regulatory structures that consider both the immediate medical benefits and the long‑range societal implications. Clinical adoption will depend on rigorous safety validation, clear ethical oversight, and international harmonization of standards for use. A future in which artificial wombs can extend viability and reduce human suffering is plausible, provided it unfolds within careful scientific, legal, and ethical guardrails.
Artificial Wombs: Redefining Neonatal Viability and Medical Ethics
Conclusion
Artificial wombs represent a paradigm shift in neonatal care, transcending traditional limits of prematurity and bringing new opportunities for life support and developmental medicine. By bridging the gap between womb and external life, these technologies may vastly improve outcomes for infants at the margins of viability while prompting reconsideration of fundamental clinical and ethical frameworks. As research approaches human application, the combined forces of medical innovation, ethical stewardship, and societal engagement will shape how artificial wombs are integrated into the future of healthcare with both scientific precision and human sensitivity.
Recommendation
Clinicians, researchers, and health policymakers should engage with artificial womb technology through a multidisciplinary lens. Prioritize robust preclinical validation and stakeholder‑informed ethical guidelines before human implementation. Establish transparent regulatory frameworks to govern clinical trials, parental consent, and risk‑benefit assessments. Integrate artificial womb research into neonatal care protocols where medically appropriate, and invest in public dialogue to address societal concerns around birth, autonomy, and reproductive equity.
Frequently Asked Questions
What are artificial wombs used for?
Artificial wombs primarily aim to support extremely premature infants by replicating womb conditions outside the body.
Are artificial wombs ready for human use?
Human clinical use is not yet standard; technology is progressing from preclinical models toward careful trial evaluation.
Do artificial wombs raise ethical concerns?
Yes, ethical debates include fetal personhood, parental rights, and the boundaries between medical support and reproductive autonomy.