Introduction
The evolution of genome editing has reached a point where precision, safety, and scalability matter more than raw capability. AI in CRISPR gene editing has become essential to meeting these demands, reshaping how genetic edits are designed, evaluated, and translated into real-world applications. While CRISPR technology introduced programmable DNA modification, its variability across genomes and cell types exposed critical limitations. AI in CRISPR gene editing resolves these challenges by introducing predictive intelligence across the entire workflow. As clinical and industrial expectations rise, AI in CRISPR gene editing now defines the difference between experimental success and deployable genetic solutions.
AI in CRISPR gene editing stands at the forefront of modern genome engineering, transforming how scientists design, predict, and apply gene editing in research and medicine.
What Is CRISPR, and Why AI Matters
CRISPR–Cas systems are programmable molecular tools that cut DNA at user-specified locations, directed by guide RNA (gRNA). While CRISPR revolutionized genetic manipulation with its simplicity and versatility, its performance varies widely across genomic contexts and cell types. Unintended edits—so-called off-target effects—remain a central safety concern in research and therapeutic settings. Artificial intelligence provides a mechanism to analyze enormous experimental datasets and identify patterns that human heuristics alone cannot detect, leading to more reliable and efficient editing outcomes.
Machine Learning Enhances Guide RNA Design
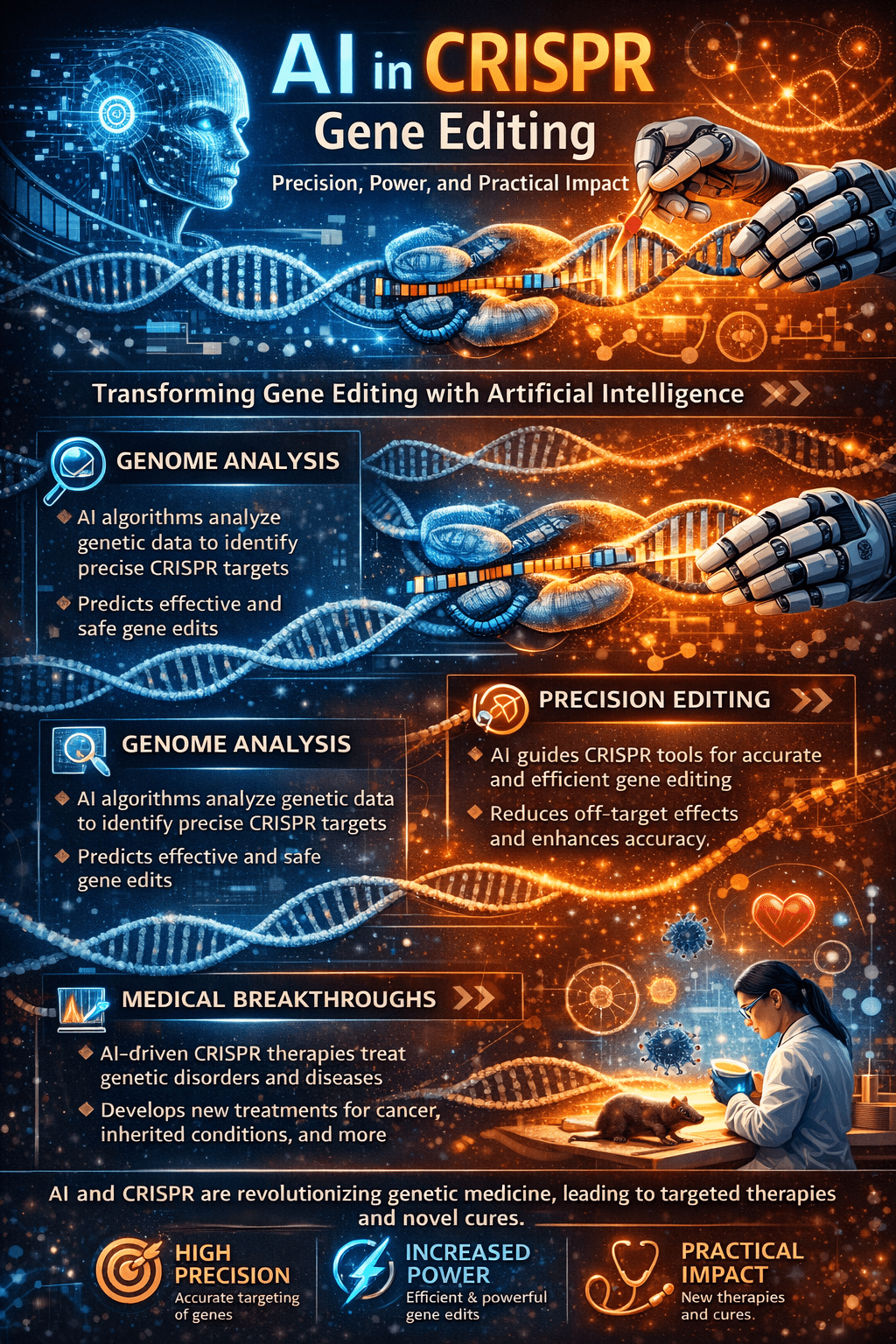
One of the most productive areas where AI impacts CRISPR is guide RNA design. The effectiveness of CRISPR editing critically depends on selecting gRNAs that will bind the intended target while avoiding similar sequences elsewhere in the genome. Traditional heuristics often fail to capture complex sequence features and chromatin context; during this process, machine learning models trained on large datasets predict gRNA activity more accurately.
Predicting and Reducing Off-Target Effects
Off-target DNA cleavage—where CRISPR cuts at unintended sites—is a fundamental risk in gene editing that can compromise safety, especially in therapeutic applications. AI-based predictors classify genomic sites by likelihood of unintended editing, using deep learning and ensemble methods to improve sensitivity and specificity beyond traditional algorithms. These tools analyze mismatch patterns, insertion/deletion tolerance, and sequence context to estimate which genomic loci might be prone to off-target activity.
Generative Models and New CRISPR Systems
Beyond optimization of existing components, generative AI models can design entirely new CRISPR effectors and related proteins. By learning sequence-to-function relationships from protein databases, these models explore sequence spaces far beyond what natural evolution samples. In early demonstrations, AI-generated gene editor variants have shown comparable on-target performance with dramatically reduced off-target activity compared to standard Cas9 enzymes. Such capacity to tailor editors for specific applications—smaller size for delivery, alternative recognition motifs, or enhanced specificity—expands the toolkit available to researchers and therapeutic developers.
Predicting Editing Outcomes and Repair Patterns
CRISPR-induced DNA breaks trigger endogenous repair pathways, which can lead to a range of outcomes, including insertions, deletions, or precise changes. Predicting the distribution of these outcomes has historically been challenging. AI models trained on empirical repair datasets can forecast likely editing products based on target site context and cell state. This predictive insight supports more strategic target selection, reducing unintended byproducts and enabling researchers to design experiments that favour desired modifications. These predictions bridge the gap between molecular cut and biological consequence.
Optimizing Delivery for Practical Use
Even with accurate design and prediction, delivering CRISPR components into living cells or tissues efficiently and safely remains a hurdle. Machine learning models applied to delivery data can predict which delivery systems—lipid nanoparticles, viral vectors, or non-viral alternatives—are most likely to succeed in a given biological context. By incorporating factors such as biodistribution, tissue barriers, and immune responses into optimization algorithms, AI helps tailor delivery strategies that complement the precision of CRISPR edits with practical, translatable methods.
Integration with Precision Medicine
The marriage of AI and CRISPR technologies underpins a new paradigm in precision medicine. Analyzing patient-specific genomic data enables bespoke editing strategies that account for unique genetic variation, improving both efficacy and safety. This personalized approach holds promise for treating genetic disorders, targeted cancer therapies, and other clinical interventions where one-size-fits-all editing is insufficient. As computational models continue to mature, the integration of genomic, phenotypic, and clinical data will deepen, making data-driven gene editing a cornerstone of next-generation therapeutics.
Challenges and Ethical Considerations
Despite impressive technical progress, integrating AI with CRISPR gene editing raises both scientific and ethical challenges. The quality and representativeness of training data directly influence model performance; biases or insufficient data can lead to misleading predictions. Additionally, as gene editing capabilities expand, particularly with AI-designed editors, ethical concerns around germline modification, equitable access, and long-term effects become increasingly salient.
Conclusion
AI in CRISPR gene editing has shifted genome engineering from artisanal experimentation toward a systematic, prediction-driven discipline. By enhancing design accuracy, reducing risks, broadening editing tools, and enabling personalized strategies, AI transforms both the science and application of CRISPR. As computational models and experimental methods co-evolve, their synergy promises to accelerate breakthroughs in genetics, biotechnology, and medicine with greater confidence and precision than ever before.
Frequently Asked Questions
How does AI improve CRISPR accuracy?
AI analyzes genomic patterns and experimental outcomes to predict which guide RNAs will work best and avoid unintended edits.
Can AI help design new CRISPR tools?
Yes. Generative AI models can create novel gene editors with tailored properties, expanding the editing toolkit.
Is AI essential for therapeutic CRISPR applications?
AI enhances safety and specificity, making genome editing more viable for clinical use, though experimental validation remains crucial.